Research content
Ocular Inflammation Group
Elucidation of pathogenesis of uveitis and development of diagnostics and optimized therapeutics by analyzing clinical specimens
P.I.;Koh-Hei Sonoda ,Atsunobu Takeda, Nobuyo Yawata
Uveitis is a diverse group of diseases, including infectious, autoimmune, autoinflammatory, and malignant diseases, with large variations. Different responses to treatment and prognoses call attention to the importance of human immunology research and the elucidation of diverse disease pathologies. In recent years, advanced technologies such as single cell analysis and next-generation sequencing (NGS) have developed, which enable us to obtain a great extent of information from a limited amount of clinical specimens. They are powerful tools in elucidating the pathogenesis and stratification of inflammatory diseases. In order to clarify the factors involved in the ocular inflammation and treatment responsiveness, and to develop diagnostic techniques and optimal therapeutics, our laboratory is analyzing intraocular fluid and blood specimens of uveitis at the gene expression, protein, and cellular levels using these state-of-the-art technologies.
We are also collaborating with uveitis treatment specialists and research institutions across the country to advance our research.
Simultaneous comprehensive analysis of uveitis specimens using Cytometry Time by Flight (CyTOF)
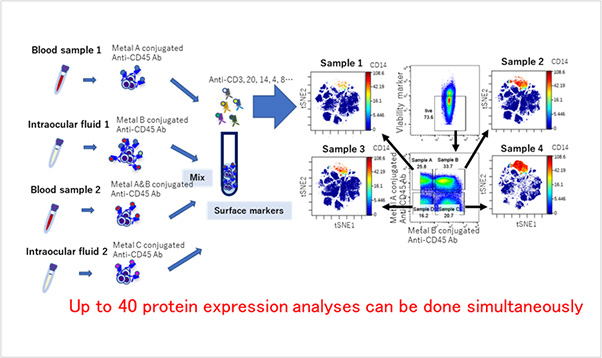
Infrequent subsets of peripheral blood mononuclear cells in the Vogt-Koyanagi-Harada
disease with persistent inflammation have been found.
Yamana et al, Mucosal Immunol 2022Analysis of cytomegalovirus gene polymorphisms in intraocular fluids and peripheral blood using NGS
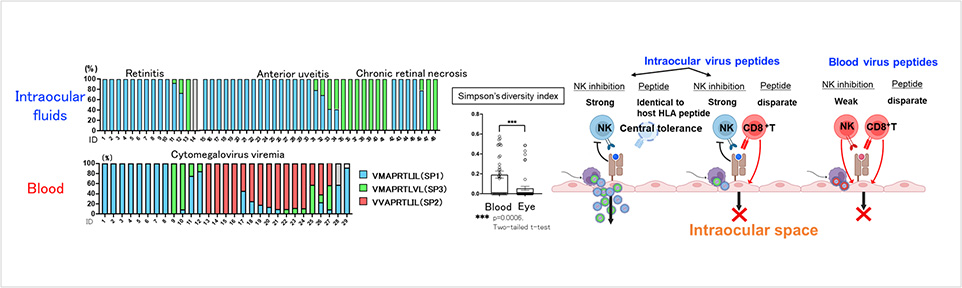
Distributions of cytomegalovirus gene polymorphisms in intraocular fluids and peripheral blood differs. Cytomegaloviruses in intraocular fluids possess higher capacity to escape both from innate and adaptive immunity utilizing HLA-E.
Shirane et al, Front Immunol 2022
Elucidation of pathogenesis of autoimmune uveitis.
Uveitis is one of the refractory eye diseases leading cause of severe loss of vision. It is defined as inflammation of the uvea, which has infectious or non-infectious causes. The precise immunologic mechanisms of uveitis with systemic or autoimmune diseases still remain unclear. Our research group has shown that both Th1 and Th17 cells are responsible for the pathogenesis of uveitis using the rodent model of uveitis (Experimental Autoimmune Uveitis: EAU). (Sonoda KH et al. Acta Ophthalmol. 2011). We recently elucidated that mucosal-associated invariant T (MAIT) cells (Yamana S et al. Mucosal Immunol. 2022) are related to the pathogenesis of uveitis. MAIT cells are an innate-like T cell subset that harbors unique invariant TRAV1-TRAJ33+ chains paired with limited T cell receptor (TCR) β chains. An EAU experiment revealed that genetic depletion of MAIT cells exacerbated retinal pathology. An intravitreal administration of an antigenic metabolite of MAIT cells into EAU mice induced retinal MAIT cell expansion, leading to an improvement in retinal pathology. Further research is currently underway to develop novel therapies for uveitis targeting MAIT cells.
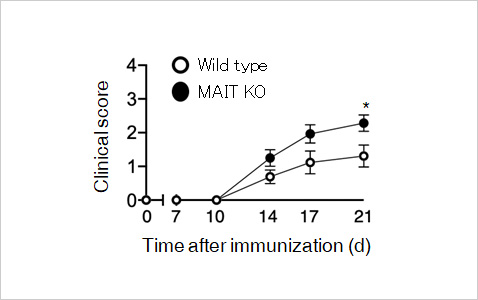
Genetic depletion of MAIT cells exacerbated retinal pathology.
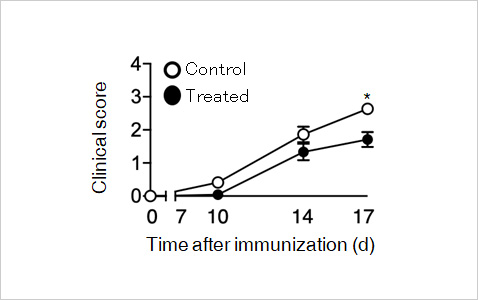
Administration of an antigenic metabolite of MAIT cells improved
retinal pathology.
Yamana et al, Mucosal Immunol 2022
Selected publications
- A multicenter study of ocular inflammation after COVID-19 vaccination.
Yasaka Y, Hasegawa E, Keino H, Usui Y, Maruyama K, Yamamoto Y, Kaburaki T, Iwata D, Takeuchi M, Kusuhara S, Takase H, Nagata K, Yanai R, Kaneko Y, Iwahashi C, Fukushima A, Ohguro N, Sonoda KH; JOIS Uveitis Survey Working Group. Jpn J Ophthalmol. 2023 Jan;67(1):14-21. doi: 10.1007/s10384-022-00962-9. - Intraocular human cytomegaloviruses of ocular diseases are distinct from those of viremia and are capable of escaping from innate and adaptive immunity by exploiting HLA-E-mediated peripheral and central tolerance.
Shirane M, Yawata N, Motooka D, Shibata K, Khor SS, Omae Y, Kaburaki T, Yanai R, Mashimo H, Yamana S, Ito T, Hayashida A, Mori Y, Numata A, Murakami Y, Fujiwara K, Ohguro N, Hosogai M, Akiyama M, Hasegawa E, Paley M, Takeda A, Maenaka K, Akashi K, Yokoyama WM, Tokunaga K, Yawata M, Sonoda KH. Front Immunol. 2022 Oct 19; 13:1008220. doi: 10.3389/fimmu.2022.1008220. - Factors associated with low prevalence of Fuchs' uveitis syndrome in Japan.
Yoneda Y, Usui Y, Tanaka R, Hase K, Namba K, Kamoi K, Takase H, Takeuchi M, Matsumiya W, Kusuhara S, Takeda A, Yawata N, Yanai R, Hiyama T, Harada Y, Hashida N, Maruyama K, Nakai K, Taguchi R, Kaburaki T, Mizuki N, Goto H, Fujino Y, Takeuchi M. Front Med (Lausanne). 2022 Sep 30; 9:999804. doi: 10.3389/fmed.2022.999804. - Long-term outcomes of infliximab in patients with Behçet's disease-associated uveitis.
Yamana S, Hasegawa E, Takeda A, Yawata N, Sonoda KH. Int Ophthalmol. 2022 Sep 4. doi: 10.1007/s10792-022-02495-z. Online ahead of print. - Pauveitis induced by donor-derived CD8+ T cells after allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia.
Takeda A, Sakoda T, Yawata N, Kato K, Hasegawa E, Shima T, Hikita S, Yoshitomi K, Takenaka K, Oda Y, Akashi K, Sonoda KH. Am J Ophthalmol Case Rep. 2022 Aug 5; 27:101673. doi:
10.1016/j.ajoc.2022.101673. - Increased vitreous levels of B cell activation factor (BAFF) and soluble interleukin-6 receptor in patients with macular edema due to uveitis related to Behçet's disease and sarcoidosis.
Takeda A, Hasegawa E, Yawata N, Notomi S, Ishikawa K, Murakami Y, Hisatomi T, Kimura K, Sonoda KH.
Graefes Arch Clin Exp Ophthalmol. 2022 Mar 1. doi: 10.1007/s00417-022-05600-1. - Mucosal-associated invariant T cells have therapeutic potential against ocular autoimmunity.
Yamana S, Shibata K, Hasegawa E, Arima M, Shimokawa S, Yawata N, Takeda A, Yamasaki S, Sonoda KH.
Mucosal Immunol. 2022 Feb;15(2):351-361. doi: 10.1038/s41385-021-00469-5. - Takeda A, Hasegawa E, Notomi S, Ishikawa K, Arima M, Murakami Y, Nakao S, Hisatomi T, Sonoda KH. Clin Ophthalmol. 2021 Jun 24; 15:2665-2673. doi: 10.2147/OPTH.S314173.
- Molecular Signatures of Natural Killer Cells in CMV-Associated Anterior Uveitis, A New Type of CMV-Induced Disease in Immunocompetent Individuals.
Yawata N, Shirane M, Woon K, Lim X, Tanaka H, Kawano YI, Yawata M, Chee SP, Siak J, Sonoda KH. Int J Mol Sci. 2021 Mar 31;22(7):3623. doi: 10.3390/ijms22073623. - Epidemiology of uveitis in Japan: a 2016 retrospective nationwide survey. Sonoda KH, Hasegawa E, Namba K, Okada AA, Ohguro N, Goto H; JOIS (Japanese Ocular Inflammation Society) Uveitis Survey Working Group. Jpn J Ophthalmol. 2021 Mar;65(2):184-190. doi: 10.1007/s10384-020-00809-1.
- Vitreous levels of interleukin-35 as a prognostic factor in B-cell vitreoretinal lymphoma.
Takeda A, Hasegawa E, Nakao S, Ishikawa K, Murakami Y, Hisatomi T, Arima M, Yawata N, Oda Y, Kimura K, Yoshikawa H, Sonoda KH.
Sci Rep. 2020 Sep 24;10(1):15715. doi: 10.1038/s41598-020-72962-z. - New insights into immunological therapy for retinal disorders.
Takeda A, Yanai R, Murakami Y, Arima M, Sonoda KH.
Front Immunol. 2020 Jul 3;11:1431. doi: 10.3389/fimmu.2020.01431. eCollection 2020. - Risk factors for failure of vitrectomy cell block technique in cytological diagnosis of vitreoretinal lymphoma.
Ito T, Takeda A, Fujiwara K, Hasegawa E, Nakao S, Ohishi Y, Oda Y, Yoshikawa H, Sonoda KH.
Graefes Arch Clin Exp Ophthalmol. 2019 May;257(5):1029-1036. doi: 10.1007/s00417-019-04266-6. - The effectiveness of adalimumab treatment for non-infectious uveitis.
Hasegawa E, Takeda A, Yawata N, Sonoda KH.
Immunol Med. 2019 Jun;42(2):79-83. doi: 10.1080/25785826.2019.1642080. - Interleukin-6 plays a crucial role in the development of subretinal fibrosis in a mouse model.
Sato K, Takeda A, Hasegawa E, Jo YJ, Arima M, Oshima Y, Ryoji Y, Nakazawa T, Yuzawa M, Nakashizuka H, Shimada H, Kimura K, Ishibashi T, Sonoda KH.
Immunol Med. 2018 Mar;41(1):23-29. doi: 10.1080/09114300.2018.1451609. - Crucial role of P2X7 receptor for effector T cell activation in experimental autoimmune uveitis.
Takeda A, Yamada H, Hasegawa E, Arima M, Notomi S, Myojin S, Yoshimura T, Hisatomi T, Enaida H, Yanai R, Kimura K, Ishibashi T, Sonoda KH.
Jpn J Ophthalmol. 2018 May;62(3):398-406. doi: 10.1007/s10384-018-0587-4. - Cytochrome P450 monooxygenase lipid metabolites are significant second messengers in the resolution of choroidal neovascularization.
Hasegawa E, Inafuku S, Mulki L, Okunuki Y, Yanai R, Smith KE, Kim CB, Klokman G, Bielenberg DR, Puli N, Falck JR, Husain D, Miller JW, Edin ML, Zeldin DC, Lee KSS, Hammock BD, Schunck WH, Connor KM.
Proc Natl Acad Sci U S A. 2017 Sep 5;114(36):E7545-E7553. doi: 10.1073/pnas.1620898114. - Epidemiology of Uveitis, Caused by HTLV-1, Toxoplasmosis, and Tuberculosis; the Three Leading Causes of Endemic Infectious Uveitis in Japan.
Takeda A, Ishibashi T, Sonoda KH.
Ocul Immunol Inflamm. 2017;25(sup1):S19-S23. doi: 10.1080/09273948.2016.1253851. Review.
Ocular Fibrosis Group
P.I.;Keijiro Ishikawa
1. Development of molecular-targeting therapies for intraocular proliferative
diseases including Diabetic retinopathy, Proliferative vitreoretinopathy and Age-related macular degeneration
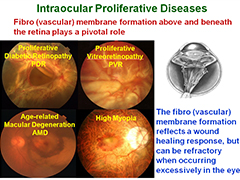
Intraocular proliferative diseases such as diabetic retinopathy (DR), age-related macular degeneration (AMD) and proliferative vitreoretinopathy (PVR) are a leading cause of decreased vision and blindness in Japan. In those diseases, retinal fibro(vascular) membrane formation above and beneath the retina plays a pivotal role in the primary pathology (Figure 1).
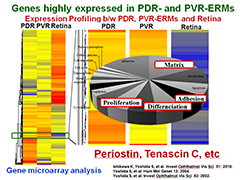
In order to identify genes responsible for intraocular proliferation, we first determined the gene expression profiling of human retina, ERMs associated with proliferative diabetic retinopathy (PDR-ERMs), and PVR (PVR-ERMs) (Figure 2). We next determined "highly expressed genes in PDR- and in PVR-ERMs" by comparing the gene expression profiles between PDR-, PVR-ERMs and the retina. Subsequent analyses identified matricellular proteins, including periostin and tenascin C as important molecules whose expressions are enhanced specifically in proliferating ERMs compared to the retina (Ishikawa K et al. IOVS 2015).
We found increased periostin and tenascin C expression in the vitreous of patients with both PDR and PVR. Immunohistochemical analysis showed colocalization of periostin and α-SMA in PDR- and PVR-ERMs (Kobayashi Y et al. Mol Vis 2016; Ishikawa K et al. FASEB J 2014; Yoshida S et al. IOVS 2012). In vitro, both periostin and tenascin C increased proliferation, adhesion, migration and collagen production in RPE cells. Periostin blockade suppressed migration and adhesion induced by transforming growth factor-β2 (TGF-β2) and PVR vitreous. In vivo, periostin and tenascin C inhibition had the inhibitory effect on experimental retinal and choroidal fibrovascular formation, and progression of experimental PVR without affecting the viability of retinal cells (Kobayashi Y et al. Lab Invest 2016; Ishikawa K et al. FASEB J 2014). These results identified periostin and tenascin C as a pivotal molecule for fibro(vascular) formation. Thus, developing the novel antibody and/or innovative could be a potential therapeutic strategy for inhibiting the progression of intraocular proliferative diseases including DR and AMD.
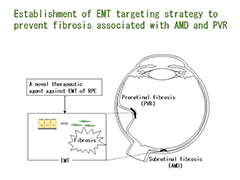
2. Exploration of the underlying mechanisms in EMT of RPE;Establishment of EMT targeting therapy to prevent fibrosis associated with AMD and PVR.
In the pathogenesis of intraocular fibrosis associated with AMD and PVR, epithelial to mesenchymal transition (EMT) of RPE is one of the important steps; that is, RPE that undergo EMT acquire a fibroblast phenotype with increased capacity to proliferate and migrate and with the ability to produce ECM, which facilitate the formation of fibrotic membrane (Ishikawa K et al. Am J Pathol. 2016). Our comprehensive gene expression analyses of surgically resected human fibrous membranes associated with PDR and PVR revealed the significant EMT-related molecules (Ishikawa K et al. IOVS 2015).
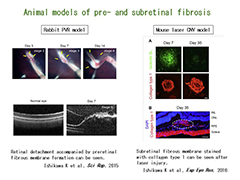
We study the underlying mechanisms in EMT of RPE by investigating the expression and functions of those EMT-related molecules in human samples and/or the animal models of both pre- and subretinal fibrosis (rabbit PVR model and mouse laser-induced CNV model) by real-time PCR, ELISA, Western blot and IHC, etc (Figure 3) (Ishikawa K et al. Sci Rep. 2015; Ishikawa K et al. Exp Eye Res. 2016). Moreover, we explore the biological function and the underlying molecular pathways related to EMT in vitro and seek to establish EMT targeting therapy to prevent fibrosis associated with AMD and PVR (Figure 4).
- Human samples or clinical information can be collected after approval by the Ethics Committee of the Kyushu University Hospital, and obtainment of informed consent for the surgery and the use of specimens from patients. The expression of the molecules can be examined using those surgically-resected specimens and vitreous samples.
- The pathways mediated by those molecules can be further examined by in vitro assay using primary RPE cells.
- Check the inhibitory effect of those molecules in both animal models, and determine whether the molecules can be novel therapeutic targets.
Selected publications
- TNFRSF10A downregulation induces retinal pigment epithelium degeneration during the pathogenesis of age-related macular degeneration and central serous chorioretinopathy
Mori K, Ishikawa K, Fukuda Y, Rui ji, Wada I, Kubo Y, Akiyama M, Notomi Shoji, Murakami Y, Nakao S, Arakawa S, Shiose Satomi, Hisatomi T, Yoshida S, Ram Kannan, Sonoda KH;
Hum Mol Genet. 2022/02/01 - Drainage retinotomy confers risk of epiretinal membrane formation following vitrectomy for rhegmatogenous retinal detachment repair
Ishikawa K, Akiyama M, Mori K, Nakama T, Notomi S, Nakao S, Kohno RI,Takeda A, Sonoda KH;
Am J Ophthalmol. 234:20-27, 2021/07/30 - Changes in metamorphopsia after the treatand-extend regimen of anti-VEGF therapy for macular edema associated with branch retinal vein occlusion.
Mori K, Ishikawa K, Wada I, Kubo Y, Kobayashi Y, Nakama T, Masatoshi Haruta, Akiyama M, Nakao S, Yoshida S, Sonoda KH.
PLoS One. 15:10, 2020/10/28 - Periostin and tenascin-C interaction promotes angiogenesis in ischemic proliferative retinopathy.
Kubo Y, Ishikawa K, Mori K, Kobayashi Y, Nakama T, Arima M, Nakao S, Hisatomi T, Haruta M, Yoshida S, Sonoda KH.
Sci Rep. 10(1):9299, 2020/06/09 - Vitrectomy with peripapillary internal limiting membrane peeling for macular retinoschisis associated with normal-tension glaucoma.
Ishikawa K, Fukui T, Nakao S, Shiose S, Sonoda KH.
Am J Ophthalmol Case Rep. 2020 Mar 17;18:100663. - Increased expression of periostin and tenascin-C in eyes with neovascular glaucoma secondary to PDR.
Ishikawa K, Kohno RI, Mori K, Murakami Y, Nakao S, Akiyama M, Yoshida S, Sonoda KH.
Graefes Arch Clin Exp Ophthalmol. 2020 Mar ;258(3):621-628. - Decrease in the number of microaneurysms in diabetic macular edema after anti-vascular endothelial growth factor therapy: implications for indocyanine green angiography-guided detection of refractory microaneurysms.
Mori K, Yoshida S, Kobayashi Y, Ishikawa K, Nakao S, Hisatomi T, Haruta M, Isihibashi T, Sonoda KH.
Graefes Arch Clin Exp Ophthalmol. 2020 Apr;258(4):735-741. - Preoperative estimation of distance between retinal break and limbus with wide-field fundus imaging: Potential clinical utility for conventional scleral buckling.
Ishikawa K, Kohno RI, Hasegawa E, Nakao S, Yoshida S, Sonoda KH.
PLoS One. 2019 Feb 12;14(2):e0212284. - αB-crystallin regulates subretinal fibrosis by modulation of epithelial-mesenchymal transition.
Ishikawa K, Sreekumar PG, Spee C, Nazari H, Zhu D, Kannan R, Hinton DR.
Am J Pathol. 2016;186:859-73. - Molecular mechanisms of subretinal fibrosis in age-related macular degeneration.
Ishikawa K, Kannan R, Hinton DR.
Exp Eye Res. 2016;142:19-25. - Tenascin-C secreted by transdifferentiated retinal pigment epithelial cells promotes choroidal neovascularization via integrin alphaV.
Kobayashi Y, Yoshida S, Zhou Y, Nakama T, Ishikawa K, Kubo Y, Arima M, Nakao S, Hisatomi T, Matsuda A, Sonoda KH, Ishibashi T.
Lab Invest. 2016;96:1178-1188. - Different roles played by periostin splice variants in retinal neovascularization.
Nakama T, Yoshida S, Ishikawa K, Kobayashi Y, Abe T, Kiyonari H, Shioi G, Katsuragi N, Ishibashi T, Morishita R, Taniyama Y.
Exp Eye Res. 2016;153:133-140. - Resveratrol inhibits epithelial-mesenchymal transition of retinal pigment epithelium and development of proliferative vitreoretinopathy.
Ishikawa K, He S, Terasaki H, Nazari H, Zhang H, Spee C, Kannan R, Hinton DR.
Sci Rep. 2015;10;5:16386. - Microarray analysis of gene expression in fibrovascular membranes excised from patients with proliferative diabetic retinopathy.
Ishikawa K, Yoshida S, Kobayashi Y, Zhou Y, Nakama T, Nakao S, Sassa Y, Oshima Y, Niiro H, Akashi K, Kono T, Ishibashi T.
Invest Ophthalmol Vis Sci. 2015;56:932-946. - Periostin promotes the generation of fibrous membranes in proliferative vitreoretinopathy.
Ishikawa K, Yoshida S, Nakao S, Nakama T, Kita T, Asato R, Sassa Y, Arita R, Miyazaki M, Enaida H, Oshima Y, Murakami N, Niiro H, Ono J, Matsuda A, Goto Y, Akashi K, Izuhara K, Kudo A, Kono T, Hafezi-Moghadam A, Ishibashi T.
FASEB J. 2014;28:131-142.
Inherited Retinal Diseases and Gene Therapy Group
P.I.;Yusuke Murakami
Toward development of new therapies against intractable retinal dystrophies
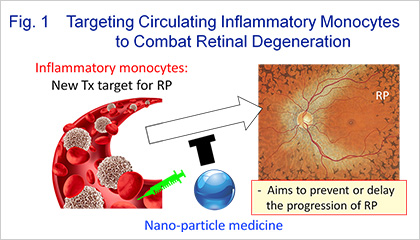
Inherited retinal diseases (IRDs) are currently incurable disorders with which more than 200 causal genes are associated. Our team aims to elucidate the genotype-phenotype relationships in IRDs as well as to clarify the mechanisms of retinal degeneration, thereby delivering new tools and medicine (e.g. gene therapy, nano-particle medicine, and novel compounds) to IRDs patients with a significant unmet needs. One major interest of our lab is the mechanisms of cell death and neuroinflammation in retinitis pigmentosa (RP), a form of IRDs with rod-cone dystrophy. For example, we have recently identified that circulating blood inflammatory monocytes contribute to the progression of RP, and we are now developing nano-particle medicine that attenuates inflammatory monocytes and peripherally engrafted macrophages (Fig. 1).
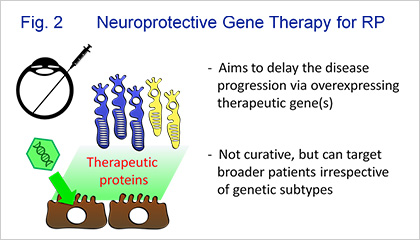
Based on our basic and clinical studies, we are now conducting Phase 1/2a clinical trial of neuroprotective gene therapy for patients with RP. In this trial, lentiviral vector encoding pigment epithelium-derived factor (PEDF) is injected into the subretinal space for the purpose of delaying the disease progression (Fig. 2). We are also developing gene therapy agents against other IRDs that are frequent in Asian populations.
Selected publications
- Circulating inflammatory monocytes oppose microglia and contribute to cone cell death in retinitis pigmentosa.
Funatsu J, Murakami Y, Shimokawa S, Nakatake S, Fujiwara K, Okita A, Fukushima M, Shibata K, Yoshida N, Koyanagi Y, Akiyama M, Notomi S, Nakao S, Hisatomi T, Takeda A, Paschalis EI, Vavvas DG, Ikeda Y, Sonoda KH.
PNAS Nexus. 2022 ; 1: pgac003. - Genotype and Long-term Clinical Course of Bietti Crystalline Dystrophy in Korean and Japanese Patients.
Murakami Y, Koyanagi Y, Fukushima M, Yoshimura M, Fujiwara K, Akiyama M, Momozawa Y, Ueno S, Terasaki H, Oishi A, Miyata M, Ikeda H, Tsujikawa A, Mizobuchi K, Hayashi T, Fujinami K, Tsunoda K, Park JY, Han J, Kim M, Lee CS, Kim SJ, Park TK, Joo K, Woo SJ, Ikeda Y, Sonoda KH.
Ophthalmol Retina. 2021; S2468-6530. - Changes of Serum Inflammatory Molecules and Their Relationships with Visual Function in Retinitis Pigmentosa
Okita A, Murakami Y, Shimokawa S, Funatsu J, Fujiwara K, Nakatake S, Koyanagi Y, Akiyama M, Takeda A, Hisatomi T, Ikeda Y, Sonoda KH;
Invest Ophthalmol Vis Sci. 61(11):30, 2020/09/01 - Aqueous Flare and Progression of Visual Field Loss in Patients With Retinitis Pigmentosa
Kohta Fujiwara , Yasuhiro Ikeda , Yusuke Murakami , Takashi Tachibana , Jun Funatsu , Yoshito Koyanagi , Shunji Nakatake , Shotaro Shimokawa , Noriko Yoshida , Shintaro Nakao , Toshio Hisatomi , Tatsuro Ishibashi , Koh-Hei Sonoda Invest Ophthalmol Vis Sci. 61(8):26.2020/7/1 - Innate immune response in retinal homeostasis and inflammatory disorders.
Murakami Y, Ishikawa K, Nakao S, Sonoda KH.
Prog Retin Eye Res. 2020 Jan;74:100778 - Genetic characteristics of retinitis pigmentosa in 1204 Japanese patients.
Koyanagi Y, Akiyama M, Nishiguchi KM, Momozawa Y, Kamatani Y, Takata S, Inai C, Iwasaki Y, Kumano M, Murakami Y, Omodaka K, Abe T, Komori S, Gao D, Hirakata T, Kurata K, Hosono K, Ueno S, Hotta Y, Murakami A, Terasaki H, Wada Y, Nakazawa T, Ishibashi T, Ikeda Y, Kubo M, Sonoda KH.
J Med Genet. 2019 Oct;56(10):662-670. - MUTYH promotes oxidative microglial activation and inherited retinal degeneration.
Nakatake S, Murakami Y, Ikeda Y, Morioka N, Tachibana T, Fujiwara K, Yoshida N, Notomi S, Hisatomi T, Yoshida S, Ishibashi T, Nakabeppu Y, Sonoda KH.
JCI Insight. 2016 Sep 22;1(15):e87781. - Clinical evidence of sustained chronic inflammatory reaction in Retinitis Pigmentosa.
Yoshida N, Ikeda Y, Notomi S, Ishikawa K, Murakami Y, Hisatomi T, Enaida H, Ishibashi T.
Ophthalmology. 2013 Jan;120(1):100-5. - Stable Retinal Gene Expression in Nonhuman Primates via Subretinal Injection of SIVagm-based Lentiviral Vectors.
Ikeda Y, Yonemitsu Y, Miyazaki M, Kohno R, Murakami Y, Murata T, Tabata T, Ueda Y, Ono F, Suzuki T, Ageyama N, Terao K, Hasegawa M, Sueishi K, Ishibashi T.
Hum Gene Ther. 2009 Jun;20(6):573-9. - Simian lentiviral vector-mediated retinal gene transfer of pigment epithelium-derived factor protects retinal degeneration and electrical defect in Royal College of Surgeons rats.
Miyazaki M, Ikeda Y, Yonemitsu Y, Goto Y, Sakamoto T, Tabata T, Ueda Y, Hasegawa M, Tobimatsu S, Ishibashi T, Sueishi K.
Gene Ther. 2003 Aug;10(17):1503-11.
Ocular Tumor and Genomics Group
Ocular Oncology Group
PIs:Mika Tanabe , and Masato Akiyama
Ocular tumor is the life-threatening disease. Our department is one of the specialized institutions for the diagnosis and treatment of ocular tumors in Japan. In the Kyushu university hospital, we treat ocular tumors including orbital tumors, lacrimal gland tumors, eyelid tumors, conjunctival tumors, and intraocular tumors. The number of new cases with ocular tumors is about 250 cases per year. As well as the clinical practice, we are conducting basic research.
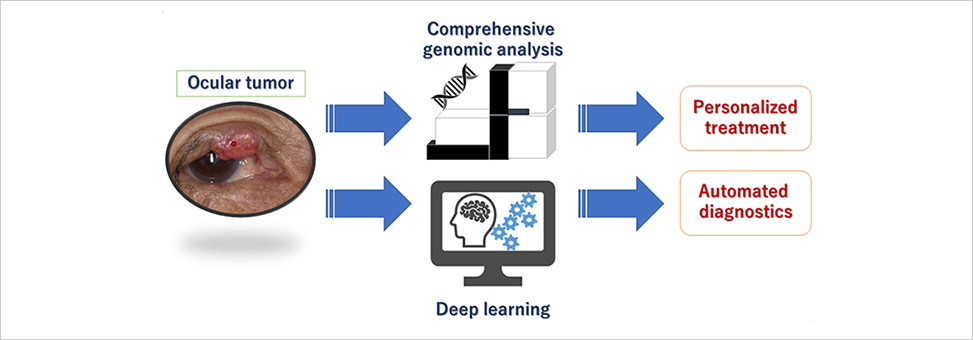
Recent advantages in genomic analysis expand our understandings of cancer genomes. These studies can provide us with a novel approach for the selection of the treatment drugs, and sub-classifications of the tumors based on molecular level information. Nevertheless, genomic analyses have been performed in a few tumors in the ophthalmologic field. We have started the genomic analysis of the ocular tumors by comprehensive DNA and RNA sequencing using the next-generation sequencer.
We have also launched a new project using the artificial intelligence (AI). Since the majority of the ocular tumors were rare, the diagnosis of ocular tumor is not easy for the family doctors and general ophthalmologists. Now, our department have the computational server which contains graphic processing units (GPU) in order to develop a deep learning system for the precise diagnosis of ocular tumors.
Selected publications
- High-risk HPV-related Squamous Cell Carcinoma in the Conjunctiva and Lacrimal sac: Clinicopathologic Characteristics and Diagnostic Utility of p16 and Rb Immunohistochemistry Hongo T, Yamamoto H, Tanabe M, Yasumatsu R, Kuga R, Miyazaki Y, Jiromaru R, Hashimoto K, Tateishi Y, Sonoda KH, Nakagawa T, Oda Y; Am J Surg Pathol. 46(7):977-987, 2022/07/01
- Two Cases of Angiosarcoma with Persistent Unilateral Eyelid Swelling
Sakisaka S, Tanabe M, Imayama S, Zeze N, Yamana K, Yoshikawa H, Sonoda KH;
Ocular Oncology and Pathology 8(1):16-21, 2022/02/01 - Defining High-Risk Retinoblastoma: A Multicenter Global Survey
Swathi K, Carol L S, Nathalie C, Francis LM, Guillermo C, Hans EG, Yoshikawa H, Ido Didi F, Jesse LB, John DM, Kahaki K, M Ashwin R, Manoj P, Tanabe M, Furuta M, Natalia G, Patricia Chevez-Barrios, Patricia S, Ralph CE Jr, Riffat R, Rosdali Díaz C, Sadia S, Sandra S, Shahar F, Suzuki S, Tatiana LU, Xunda Ji;
JAMA ophthalmology. 140(1):30-36, 2022/01/01 - Alveolar soft part sarcoma of the orbit: A case report
Oda T, Kikuchi K, Togao O, Baba S, Mizoguchi M, Tanabe M, Itoiu M, Yamamoto H, Ishigami K, Hiwatashi A;
Radiology Case reports. 16:3766-3771, 2021/09/05 - A case of primary orbital solitary fibrous tumor with lung metastases 41 years after initial treatment
Tanabe M, Yoshikawa H, Yuichi Yamada, Yoshinao Oda, Sonoda KH;
Orbit. 10:1-5, 2021/07/14 - Survival and ocular preservation in a long-term cohort of Japanese patients with retinoblastoma.
Ueda T, Koga Y, Yoshikawa H, Tanabe M, Yamana K, Oba U, Nakashima K, Ono H, Ichimura T, Hasegawa S, Kato W, Kobayashi T, Nakayama H , Sakai Y, Yoshitake T, Ohga S, Oda Y, Suzuki S, Sonoda KH, Ohga S
BMC Pediatr. 2020 Jan 28;20(1):37, - Cataracts after Low-Dose Radiotherapy for Lymphoproliferative Disease of the Ocular Adnexa.
Hashimoto S, Yoshikawa H, Miyagi M, Onishi Y, Ohga S, Asai K, Ishibashi T.
Semin Ophthalmol. 2016 Jul 15:1-5. - Clinical Features of Systemic Metastatic Retinal Lymphoma in Japanese Patients.
Taki R, Takeda A, Yoshikawa H, Fukuhara T, Arita R, Suehiro Y, Choi I, Kumano Y, Nakamura T, Ishibashi T.
Ocul Immunol Inflamm. 2016 Apr 12:1-9. - Distinct Profiles of Soluble Cytokine Receptors Between B-Cell Vitreoretinal Lymphoma and Uveitis.
Takeda A, Yoshikawa H, Fukuhara T, Hikita S, Hijioka K, Otomo T, Arita R, Hisatomi T, Kimura K, Yoshida S, Kawano Y, Sonoda KH, Ishibashi T.
Invest Ophthalmol Vis Sci. 2015 Nov;56(12):7516-23. doi: 10.1167/iovs.15-17465. - Ocular surface squamous neoplasia: analysis of 34 cases.
Tanabe M, Yoshikawa H, Onishi Y, Kohno R, Ishibashi T.
Nippon Ganka Gakkai Zasshi. 2014 May;118(5):425-32. Japanese. - A case of Muir-Torre syndrome with multiple cancers of bilateral eyelids and breast.
Kamisasanuki T, Uchino E, Fukushima J, Yoshikawa H, Ishibashi T, Sakamoto T.
Korean J Ophthalmol. 2013 Jun;27(3):204-7. doi: 10.3341/kjo.2013.27.3.204. - A case of metastatic choroidal tumor simulating a choroidal melanoma.
Arima M, Yoshikawa H, Kagimoto T, Kohno R, Ishibashi T.
Jpn J Ophthalmol. 2011 May;55(3):312-4. doi: 10.1007/s10384-011-0015-5. - Congenital simple hamartoma of the retinal pigment epithelium in an Asian.
Gotoh M, Yoshikawa H, Kagimoto HT, Ishibashi T.
Jpn J Ophthalmol. 2008 Mar-Apr;52(2):144-5. doi: 10.1007/s10384-008-0509-y. - Retinal capillary hemangioma managed by transpupillary thermotherapy.
Mochizuki Y, Noda Y, Enaida H, Hata Y, Ueno A, Yoshikawa H, Ishibashi T.
Retina. 2004 Dec;24(6):981-4. - Bilateral epiretinal membranes in nevoid basal cell carcinoma syndrome.
Yoshida S, Yoshikawa H, Yoshida A, Nakamura T, Noda Y, Gondoh H, Fukagawa S, Moroi Y, Urabe K, Furue M, Ishibashi T.
Acta Ophthalmol Scand. 2004 Aug;82(4):488-90. -
Conjunctival keratoacanthoma in an Asian.
Kifuku K, Yoshikawa H, Sonoda KH, Kawano Y, Miyazaki K, Ishibashi T.
Arch Ophthalmol. 2003 Jan;121(1):118-9.
Hisayama Ophthalmic Epidemiology Group
P.I.;K Fujiwara , E Ueda
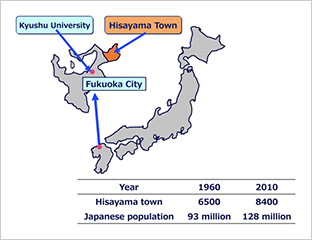
The town of Hisayama is a suburb of Fukuoka city in Japan. The population rate increase of Japan from 1960 to 2010 is similar to that of Hisayama. Age distribution was almost the same across all age categories in both 1960 and 2010, similar to that of all of Japan. Moreover, distribution of the labor population was also similar between Japan nationally and Hisayama. Therefore, we can say that Hisayama’s population is a good sample for Japan as a whole.
The Hisayama Study is an ongoing, long-term cohort study on cardiovascular disease and its risk factors in the town of Hisayama. As a part of the overall study, an epidemiologic study of eye disease among residents of the town has been under way since 1998.The purpose of our group is to clarify the prevalence, incidence and risk factors for common ocular disease, which cause visual impairment and vision loss, in a general Japanese population.
We started a new glaucoma incidence study from 2012-2013 in partnership with Oita and Akita universities. Several systemic and environmental factors related with the incidence of glaucoma are expected to be revealed. Furthermore, focusing on the potential of retinal imaging technology leading to early detection and diagnosis of dementia, we conduct to examine the relationship between the retina and the brain, and confirm its utility in community-dwelling elderly.
Selected publications
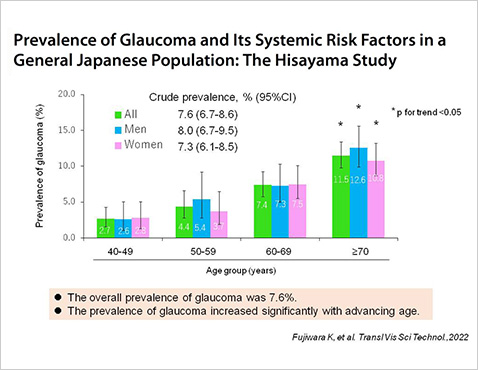
- Prevalence of glaucoma and its systemic risk actors in a General Japanese Population: The Hisayama Study. Fujiwara K, Yasuda M, Hata J, Nakano S, Hashimoto S, Ueda E, Nakamura S, Murakami Y, Nakamuro T, Iwase A, Araie M, Tawara A, Kubota T, Yoshitomi T, Ninomiya T, Sonoda KH. Transl Vis Sci Technol. 2022;11:11.
- Secular trends in the prevalence, incidence, and progression of diabetic retinopathy: the Hisayama Study Hashimoto S, Yasuda M, Fujiwara K, Ueda E, Nakamura S, Hirakawa Y, Higashioka M, Hata J, Ninomiya T, Sonoda KH; Graefes Arch Clin Exp Ophthalmol. 2022/09/24
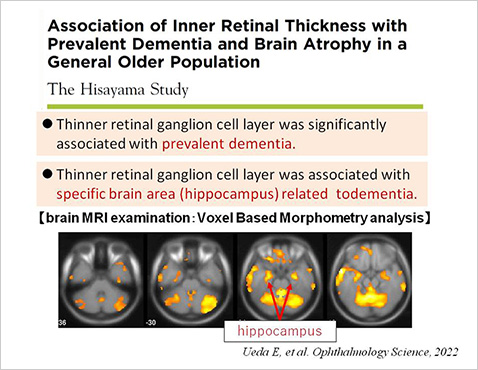
- Association of inner retinal thickness with prevalent dementia and brain atrophy in a general olderly population: the Hisayama Study Ueda E, Fujiwara K, Hashimoto S, Nakamura S, Sonoda KH; Ophthalmol Sci . 2(2):100157, 2022/04/19
- The Ganka-Ekigaku Network (GEN), a network of Japanese ophthalmological epidemiology studies.
Sasaki M, Miyake M, Fujiwara K, Nanba H, Akiyama M, Yanagi Y, Harada S, Tabara Y, Yasuda M, Yamashita H, Kayama T, Tsubota K, Matsuda F, Hashimoto S, Ueda E, Ninomiya T, Takebayashi T, Tsujikawa A, Sonoda KH, Kawasaki R:
Cohort Profile: Ophthalmic Epidemiol. 2021 Jun;28(3):237-243. - Five-year incidence of myopic maculopathy in a general Japanese population: the Hisayama Study.JAMA Ophthalmology.
Ueda E, Yasuda M, Fujiwara K, Hashimoto S, Ohno-Matsui K, Hata J, Ishibashi T, Ninomiya T, Sonoda KH.
JAMA Ophthalmol. 2020 Aug 1;138(8):887-893. - Prevalence and Pattern of geographic atrophy in Asia: the Asian Eye Epidemiology Consortium.
Tyler Hyungtaek R, Kawasaki R, Yih-Chung T, Se Woong K, Paisan R, Mukharram M. B, Miyake M, Jie H, Astrid F, Sasaki M, Vinay N, Charumathi S, Marco Y, Fujiwara K, Raba T, Ian Y W, Kayama T, Shih-Jen C, Tung-Mei K, Yamashita H, Periasamy S, Jonathan C. C, G.H. M. B van R, Sonoda KH, Ya Xing W, Songhomitra Panda-J, Sei H, Ramasamy K, Suganeswari G, Rajiv R, Kenji Y, Timur R.G, Watanee J, Kyu Hyung P, Chui Ming G, Tien Yin W, Ningli W, Jost B.J, Usha C, Ching-Yu C, Yanagi Y Ophthalmology. 2020 Oct;127(10):1371-1381. - Trends in the prevalence of myopia and myopic maculopathy in a Japanese population: the Hisayama Study.
Ueda E, Hashimoto S, Fujiwara K, Sonoda KH, Yasuda M.
Invest Ophthalmol Vis Sci. 2019 Jul;1;60(8):2781-86. - Glucose tolerance levels and circumpapillary retinal nerve fiber layer thickness in a general Japanese population: the Hisayama Study.
Fujiwara K, Yasuda M, Hata J, Hirakawa Y, Hashimoto S, Ueda E, Iwase A, Araie M, Yoshitomi Takeshi, Ninomiya T, Sonoda KH. Am J Ophthalmol.
2019 Sep;205:140-6. - Association between axial length and myopic maculopathy: the Hisayama Study.
Hashimoto S, Yasuda M, Fujiwara K, Ueda E, Hata J, Hirakawa Y, Ninomiya T, Sonoda KH.
Ophthalmology Retina. 2019 Oct;3(10):867-73. - Insulin resistance is a risk factor for increased intraocular pressure: the Hisayama Study.
Fujiwara K, Yasuda M, Ninomiya T, Hata J, Hashimoto S, Yoshitomi T, Kiyohara Y, Ishibashi T.
Invest Ophthalmol Vis Sci. 2015 Dec;56(13):7983-7. - Prevalence and risk factors for myopic retinopathy in a Japanese population: the Hisayama Study.
Asakuma T, Yasuda M, Ninomiya T, Noda Y, Arakawa S, Hashimoto S, Ohno-Matsui K, Kiyohara Y, Ishibashi T.
Ophthalmology. 2012 Sep;119(9):1760-5. - Global prevalence and major risk factor of diabetic retinopathy.
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, RoyMS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY; Meta-Analysis for Eye Disease (META-EYE) Study Group.
Diabetes Care. 2012 Mar;35(3):556-64. - Genome-wide association study identifies two susceptibility loci for exudative age-related macular degeneration in the Japanese population.
Arakawa S, Takahashi A, Ashikawa K, Hosono N, Aoi T, Yasuda M, Oshima Y, Yoshida S, Enaida H, Tsuchihashi T, Mori K, Honda S, Negi A, Arakawa A, Kadonosono K, Kiyohara Y, Kamatani N, Nakamura Y, Ishibashi T, Kubo M .
Nat Genet. 2011 Sep 11;43(10):1001-4. - Nine-year incidence and risk factors for retinal vein occlusion in a general Japanese population: the Hisayama Study.
Arakawa S, Yasuda M, Nagata M, Ninomiya T, Hirakawa Y, Doi Y, Kiyohara Y, Ishibashi T.
Invest Ophthalmol Vis Sci. 2011 Jul;52(8):5905-9. - High serum bilirubin levels and diabetic retinopathy: The Hisayama Study.
Yasuda M, Kiyohara Y, Hata Y, Arakawa S, Yonemoto K, Doi Y, Iida M, Ishibash T.
Ophthalmology. 2011 Jul;118(7):1423-8. - Prevalence and systemic risk factors of retinal vein occlusion in a general Japanese population: The Hisayama Study.
Yasuda M, Kiyohara Y, Hata Y, Arakawa S, Yonemoto K, Doi Y, Iida M, Ishibash T.
Invest Ophthalmol Vis Sci. 2010 Jun;51(6):3205-9. - Nine-year incidence and risk factors for age-related macular degeneration in a defined Japanese population: The Hisayama Study.
Yasuda M, Kiyohara Y, Hata Y, Arakawa S, Yonemoto K, Doi Y, Iida M, Ishibash T.
Ophthalmology. 2009 Nov;116(11):2135-40. - The five-year incidence and risk factors for age related maculopathy in a general Japanese population: the Hisayama Study.
Miyazaki M, Kiyohara Y, Yoshida A, Iida M, Nose Y, Ishibash T.
Invest Ophthalmol Vis Sci. 2005 Jun;46(6):1907-10. - Comparison of diagnostic methods for diabetes mellitus based on prevalence of retinopathy in a Japanese population: The Hisayama Study.
Miyazaki M, Kubo M, Kiyohara Y, Okubo K, Nakamura H, Fujisawa K, Tokunaga S, Iida M, Nose Y, Ishibashi T.
Diabetologia. 2004 Aug;47(8):1411-5.
Nerve Regeneration Group
P.I. Koh-Hei Sonoda, Yusuke Murakami, and Mitsuru Arima
It is a dream of an ophthalmologist to regenerate photoreceptor cells damaged by retinal diseases such as retinal detachment and diabetic retinopathy.
In recent years retinal regenerative medicine using iPS cells has attracted much attention, but we aim to develop retinal regeneration therapy by direct reprogramming, i.e. nerve regeneration therapy not dependent on cell transplantation. The strategy of future retinal regeneration therapy that we consider is to perform radical therapy for the causative disease and retinal regeneration therapy at the same time by injection of medicine into the vitreous at the end of surgery.
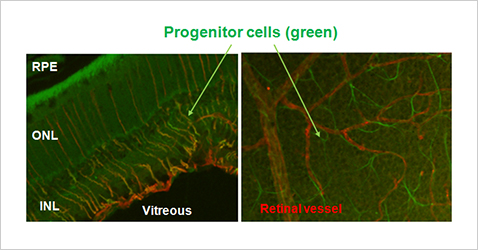
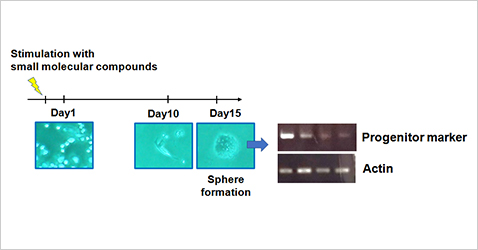
We have already developed a method to change astrocytes and Müller cells into progenitor cells by stimulation with a cocktail of small molecular compounds under in vitro environment and then induce differentiation into neurons. Now we are conducting comprehensive compound screening to select the combination of cocktails that lead to more efficient differentiation into neuronal cells.
Young researchers interested in our research, please contact us at any time!
Selected publications
- Rhodopsin-positive cell production by intravitreal injection of small molecule compounds in mouse models of retinal degeneration Fujii Y, Arima M, Murakami Y, Sonoda KH; PLoS One. 18(2):e0282174, 2023/02/23
- Changes in metabolic proteins in ex vivo rat retina during glutamate-induced neural progenitor cell induction.
Tokuda K, Kuramitsu Y, Baron B, Kitagawa T, Tokuda N, Kobayashi M, Kimura K, Sonoda KH, Nakamura K.
Mol Cell Biochem. 2016 Aug;419(1-2):177-84. - Up-regulation of DRP-3 long isoform during the induction of neural progenitor cells by glutamate treatment in the ex vivo rat retina.
Tokuda K, Kuramitsu Y, Byron B, Kitagawa T, Tokuda N, Kobayashi D, Nagayama M, Araki N, Sonoda KH, Nakamura K.
Biochem Biophys Res Commun. 2015 Aug 7;463(4):593-9.